Caris Life Sciences was founded with a very simple but powerful purpose – to help improve the lives of as many people as possible. With transformative technologies, we are revolutionizing precision medicine to provide physicians and patients with the highest quality information about their health. Through tireless efforts, breakthrough molecular science and a passionate commitment to quality, we remain steadfastly focused on the most important part of our work – the patient.”. As the pioneer in precision medicine, Caris is ushering in a new era of cancer care with blood-based monitoring for patients before treatment, during treatment and after treatment. Currently available within Caris’ Precision Oncology Alliance, our pan-cancer, circulating nucleic acids sequencing (cNAS) assay combines comprehensive molecular analysis (Whole Exome and Whole Transcriptome Sequencing from blood) and serial monitoring – making it the most powerful liquid biopsy assay ever developed.
Irving, United States
Founded in 1996
1001-5000 Employees
Working industry
Biotechnology
Type of company
Manufacturer, Service provider, Educational institution
Ownership structure
Privately Held
Locations
3 Locations
Number of products
14 Products
Number of services
2 Services
Specialised areas
Scientific Research and Development Services, Panomics, Personalized Medicine, Professional, Scientific, and Technical Services, Comprehensive Genomic Profiling, Biotechnical research, commercial, Health Diagnostics, Precision Medicine, Diagnostics, Molecular Profiling
Caris Life Sciences offers a wide range of products and services
Product
Molecular Profiling Testing Menu | Caris Life Sciences
Go to product >

Product
Caris Biopharma Discovery | Caris Life Sciences
Go to product >

Service
Financial Services | Caris Life Sciences
Go to product >

Product
Clinical Trial Solutions | Caris Life Sciences
Go to product >
Product
MI Trials | Caris Life Sciences
Go to product >
Product
Biopharma Partnering | Caris Life Sciences
Go to product >

Service
Financial Assistance Request Form | Caris Life Sciences
Go to product >
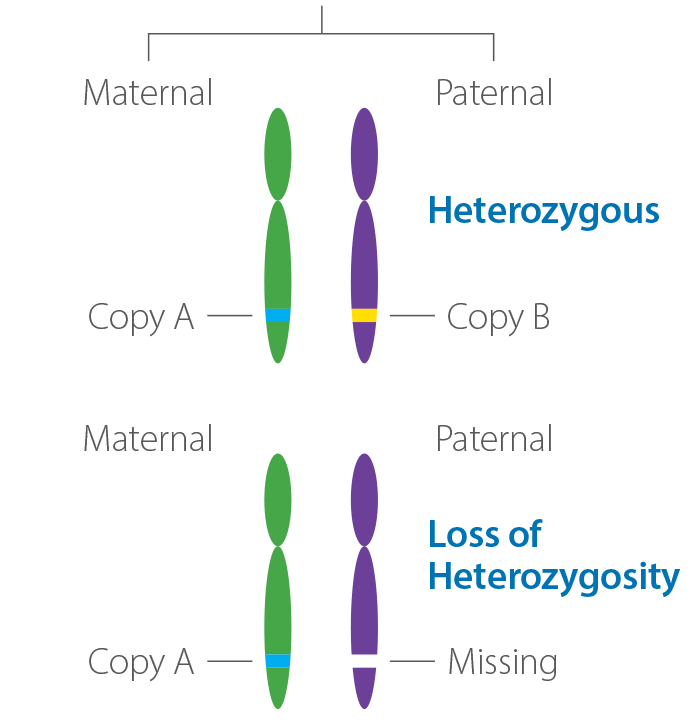
Product
Loss of Heterozygosity | Caris Life Sciences
Go to product >
An estimation about the ESG values based on digital data and signals. Important: The ESG scores are only based on information about the country, not the actual company itself
Country:
United States
Overall risk estimation:
Low
The ESG Data of countries are based on public sources
Environment
D
Grade (A-E)
View details
Social
A
Grade (A-E)
View details
Governance
A
Grade (A-E)
View details
Caris Life Sciences operates in 2 countries around the world
Get an overview of the locations of Caris Life Sciences
Location
Country
State
City
Headquarter
United States
Texas
Irving
Arizona Office/Labs
United States
Arizona
Phoenix
European Office (Basel)
Switzerland
Basel-City
Basel
Some frequent questions that have been asked about Caris Life Sciences
Where is Caris Life Sciences located?
The company headquarter of Caris Life Sciences is located in Irving, Texas, United States. Caris Life Sciences has subsidiaries in United States, Switzerland
How many employees does Caris Life Sciences approximately have?
As of the latest available information Caris Life Sciences has around 1001-5000 employees worldwide.
When was Caris Life Sciences founded?
Caris Life Sciences was founded in 1996
In which industries does Caris Life Sciences mainly work?
The company Caris Life Sciences has it's main focus in the industries of Biotechnology
Check out some interesting alternative companies to Caris Life Sciences
Pharmatech
Denver, United States
11-50 Employees
1987
We are the change and it is happening now. We are pushing the boundaries of what is possible and leading a revolution in healthcare. Nested deep in our molecular coding, is a universe of biological networks that provide the source of life. This convergence of sequencing power, big data and AI technologies provides an unmatched resource to develop the next-generation of precision medicine tools for early detection, diagnosis, monitoring, therapy selection and drug development. With a primary focus on cancer, Caris has built a market-leading portfolio of precision medicine tools that have helped more than half a million cancer patients worldwide. Patients within the Caris RIT oncology network gain access to innovative molecular diagnostics while remaining in the care of their current treatment team, locally. Having clinical trials on-demand in TrialPlus+ connects physicians to clinical trials to deliver breakthrough cancer medicines to their patients.
Universal Diagnostics
Seville, Spain
11-50 Employees
2012
We are driven forward by a vision of a future where cancer is curable. We are developing blood tests that detect cancer in its earliest stages so we can transform cancer into a disease that’s preventable and curable. We are applying our multi-omics, computational biology and machine learning approach to capture cancer’s signal for high-burden gastrointestinal cancers. Starting with colorectal cancer, we are building a multi-cancer platform that can identify the unique DNA regions associated with different types of cancers. Cancer’s early detection has become one of the greatest challenges of modern medicine, so we decided to team up with the best. Our company was founded on Silicon Valley’s ideals of technology and development (Singularity University), and we developed a network of world-class R&D partners including Quest Diagnostics and Hologic (Diagenode). Our Scientific Advisory Board includes leading oncologists, gastroenterologists and business experts from the US and Europe. We have built a network of >100 hospitals in the U.S.
Cellaria
Wakefield, United States
1-10 Employees
2013
Cellaria is a scientific innovator that is revolutionizing cancer research, patient testing, and treatment development. For scientists and researchers both in academia and industry, our new patient-specific disease models represent a true breakthrough to their important work. Now, they are empowered to move away from outdated testing models that do not reflect the complex, individualized nature of cancer and embrace a more effective and informative approach. Recognizing that every patient’s cancer is unique, our team uses a lens that highlights the differences in disease progression and treatment modalities. Ultimately, our mission is to characterize the disease models to identify treatments that better meet the needs of each patient.
DiaCarta
Hayward, United States
11-50 Employees
2011
At DiaCarta, our mission is to improve the lives of cancer patients through innovative precision diagnostics. We are committed to providing accurate, reliable, and affordable diagnostics that help physicians make informed treatment decisions. Patient-Centric operations: Our mission is to provide innovative and affordable solutions for cancer patient management, and we prioritize the needs of patients and their families in everything we do. Innovation: We are committed to advancing precision diagnostics and improving patient outcomes through cutting-edge research and development. Continuous Improvement: We are dedicated to ongoing improvement and innovation, and we invest in the development of our team, our products, and our infrastructure to better serve our customers and achieve our mission. We are committed to designing and manufacturing products with the good of the patient in mind. DiaCarta’s RadTox™ Test to Monitor Tumor Response Receives Medicare Coverage. DiaCarta Announces Successful Completion of Oncuria® Validation Study with Nonagen Bioscience.
Rarecells
Paris, France
11-50 Employees
2010
Rarecells has been working for more than 10 years in the field of blood analysis targeting early cancer detection. The ISET® technology enables Rarecells to leverage detection of those early signals into life-saving cancer tests. Rarecells is also partnering with diagnostic and pharma companies to bring its innovative tests to patients. Rarecells has a talented and dedicated team who is developing highly sensitive cancer tests that identify and analyze cancer cells. At Rarecells, we are physicians and scientists using clinical experience and scientific first-in-class innovation to develop life-saving tests and bring them to patients. Today, the cancer problem is a race against time. The early diagnosis solution to the “cancer” problem lies in innovative “liquid biopsy” technology. This strategy requires the most advanced and innovative bio-molecular techniques, which are at the core of Rarecells development work.
Pangaea Oncology
Barcelona, Spain
- Employees
-
We are specialists in finding these molecular alterations & in developing diagnostic technology, among others Liquid Biopsy. PANGAEA Oncology is a company focused on Precision Oncology, which offers both patients and pharmaceutical companies different services with the aim of improving the survival of cancer patients, their response to treatments, and their quality of life. The company has two main areas of activity interrelated, such as the clinical care division and the state-of-the-art laboratory that provides services in various areas, including molecular diagnostics as a reference laboratory for both patients and industry, pre-treatment services (clinic to industry), and diagnostic consultancy. PANGAEA ONCOLOGY’s mission is to change the natural history of the disease, improving the survival of cancer patients, through the application of the best professionals and technologies. To position PANGAEA ONCOLOGY as the reference in Europe in Precision Oncology, from patient treatment, the provision of services to the industry, the incorporation of disruptive technology and medical-scientific excellence, and directed towards systems intelligent IT and data exploitation (IA/BIG DATA). PANGAEA ONCOLOGY is a company focused on Precision Oncology, which offers both patients and pharmaceutical companies different services with the aim of improving the survival of cancer patients, their response to treatments, and their quality of life. Position PANGAEA ONCOLOGY as the reference in Southern Europe in Precision Oncology, from patient treatment, the provision of services to the industry, the incorporation of disruptive technology and medical-scientific excellence, and directed towards systems intelligent IT and data exploitation (IA/BIG DATA). Molecular companion diagnostics tests provide essential information that enables doctors to identify those patients that are most likely to benefit from a treatment.