CIRCULOGENE is on its way to becoming a top-tier biotech company by combining great talent and state-of-the-art laboratory equipment to address unmet needs for liquid biopsy. CIRCULOGENE will lead the transformation of cancer into a manageable disease. CIRCULOGENE’s liquid biopsy services leverage proven technology and provide a noninvasive, cost-effective solution for high-sensitivity detection across a variety of samples. CIRCULOGENE begins some tests with a proprietary enrichment process resulting to increase the yield of cfDNA and preserve fragile cfRNA yields. CIRCULOGENE’s PD-L1 has also been demonstrated to provide valuable information in non-small cell lung cancer (NSCLC) immunotherapy patients. CIRCULOGENE utilizes a MSI protocol which consists of eight markers – four of the gold standard markers, plus four novel markers. CIRCULOGENE then provides referenced information on current FDA-approved treatment options proven effective for the tumor DNA mutations and RNA fusions identified. We have provided state-of-the-art results to over 25,000 cancer patients since our inception.
Birmingham, United States
Founded in 2015
11-50 Employees
Working industry
Biotechnology
Type of company
Manufacturer
Ownership structure
Privately Held
Locations
1 Headquarter
Number of products
6 Products
Specialised areas
Biotechnology, Genetics
CirculoGene Theranostics offers a wide range of products and services

Product
ImmunoClear (Plasma PD-L1) | Circulogene | Liquid Biopsy Center
Go to product >

Product
Hereditary Panels | Circulogene | Tumor Detection Center
Go to product >

Product
TumorClear | Circulogene | Tumor Detection
Go to product >

Product
MSI Complete | Circulogene | Cancer Detection Center
Go to product >

Product
Products | Circulogene | Birmingham, AL
Go to product >
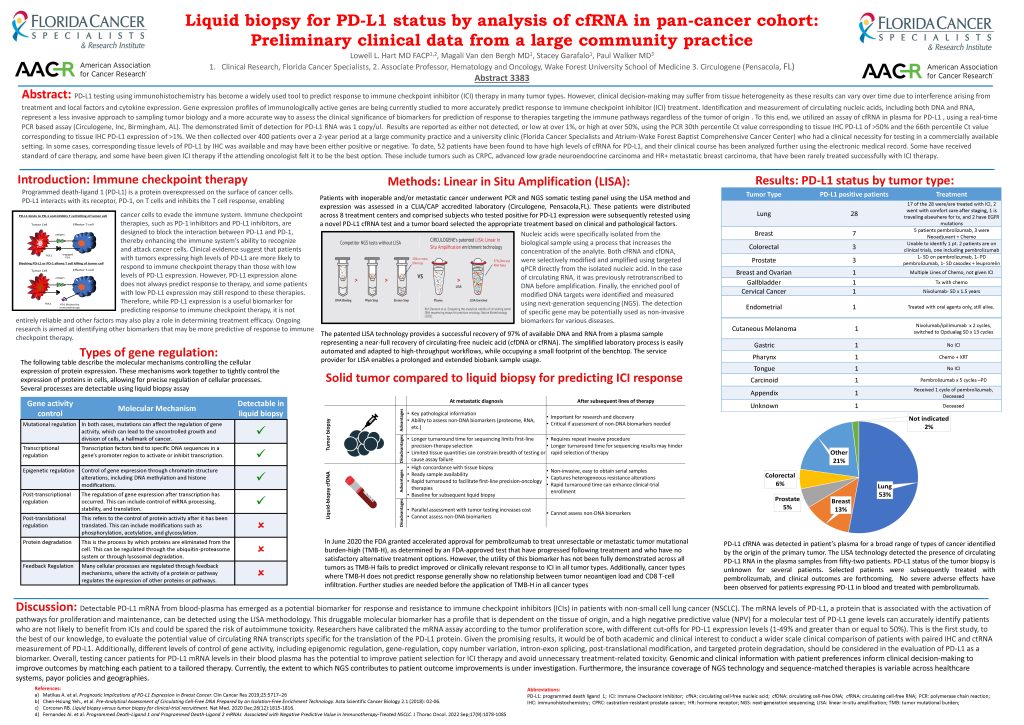
Product
CIRCULOGENE’s liquid biopsy for PD-L1 status features in research presented at the recent AACR annual meeting - Circulogene
Go to product >
An estimation about the ESG values based on digital data and signals. Important: The ESG scores are only based on information about the country, not the actual company itself
Country:
United States
Overall risk estimation:
Low
The ESG Data of countries are based on public sources
Environment
D
Grade (A-E)
View details
Social
A
Grade (A-E)
View details
Governance
A
Grade (A-E)
View details
CirculoGene Theranostics operates in 1 country around the world
Get an overview of the locations of CirculoGene Theranostics
Location
Country
State
City
Headquarter
United States
Alabama
Birmingham
Some frequent questions that have been asked about CirculoGene Theranostics
Where is CirculoGene Theranostics located?
The company headquarter of CirculoGene Theranostics is located in Birmingham, Alabama, United States. It's worth noting, that the company may have more locations
How many employees does CirculoGene Theranostics approximately have?
As of the latest available information CirculoGene Theranostics has around 11-50 employees worldwide.
When was CirculoGene Theranostics founded?
CirculoGene Theranostics was founded in 2015
In which industries does CirculoGene Theranostics mainly work?
The company CirculoGene Theranostics has it's main focus in the industries of Biotechnology
Check out some interesting alternative companies to CirculoGene Theranostics
GENECAST
Seoul, South Korea
11-50 Employees
2016
GENECAST is a company specialized in ctDNA liquid biopsy. Our outstanding detection sensitivity Improves cancer management systems, Provides more cancer treatment options, Results in a higher success rate of targeted therapy development.
Cyclomics
Utrecht, Netherlands
1-10 Employees
2018
We are a Dutch innovative health-tech company founded in 2018 by scientists of the UMC Utrecht. CyclomicsSeq is a novel ctDNA detection assay based on the latest long-read sequencing platforms. Cyclomics has developed a ground-breaking solution that enables reliable, fast and ultra- sensitive detection of cancer recurrence. To this end, Cyclomics is developing CyclomicsSeq TP53: an in vitro diagnostic (IVD) kit integrating an innovative sequencing methodology and state-of- the-art analysis software package. Cyclomics uses proprietary DNA adapters (backbones) and enzymes blends to capture ctDNA molecules and circularize them. Delivering superior performance to standard radio-logical and/ physical examination alone. In contrast to available ctDNA-based methods, CyclomicsSeq ensures that even a single ctDNA molecule present in blood can be detected and read. In the long-term, we will expand the Cyclomics’ solution to other cancer types harboring TP53 mutations.
CircaGene
London, United Kingdom
1-10 Employees
2018
At CircaGene, we believe in the importance of knowledge as a foundational element of the human being, and we have made it available to everyone. We are scientist founders looking for health improvement in the world! We are a gold standard for DNA analysis and processing. We are the future of genetic testing providing hyper-personalised genetic analysis to individuals, enabling them to proactively manage their health, and make informed decisions about their lifestyle with accurate and scientifically validated information. At CircaGene, we are pioneers in facilitating genetic services with DNA Privacy. We are the industry's unique leaders in DNA hyper-personalised analysis and Health Data management to implement distinctive patented new encryption for health data. We provide DNA analysis that supports better prognosis and treatments, as it is personalised to your specific body, in turn improving patient outcomes. CircaGene is the most complete genetic analysis, analysing ~100% of your DNA for better accuracy and using unique technological tools to protect your privacy.
Universal Diagnostics
Seville, Spain
11-50 Employees
2012
We are driven forward by a vision of a future where cancer is curable. We are developing blood tests that detect cancer in its earliest stages so we can transform cancer into a disease that’s preventable and curable. We are applying our multi-omics, computational biology and machine learning approach to capture cancer’s signal for high-burden gastrointestinal cancers. Starting with colorectal cancer, we are building a multi-cancer platform that can identify the unique DNA regions associated with different types of cancers. Cancer’s early detection has become one of the greatest challenges of modern medicine, so we decided to team up with the best. Our company was founded on Silicon Valley’s ideals of technology and development (Singularity University), and we developed a network of world-class R&D partners including Quest Diagnostics and Hologic (Diagenode). Our Scientific Advisory Board includes leading oncologists, gastroenterologists and business experts from the US and Europe. We have built a network of >100 hospitals in the U.S.
Genomill Health
Turku, Finland
1-10 Employees
2016
We are a pioneering precision diagnostics company with focus on improving liquid biopsies with our patented and proprietary molecular detection and quantification platform. Genomill’s vision is to be a leader in the transformation of precision diagnostics with the ultimate goal of enabling the full potential of liquid biopsies across the world. Our mission is to enable accurate, affordable and fast molecular diagnostics on a global scale. We are at the forefront of pioneering diagnostics, and we’re excited to share our narrative. Geno1® platform was tested by an independent laboratory, demonstrating equal performance with leading liquid biopsy technologies. Aligned Precision: Geno1®’s detection of mutant allele fractions aligns closely with established industry-standard technologies such as Illumina AmpliSeq and ArcherDX LIQUIDPlex. Trusted Verification: Independent testing confirms Geno1’s high-level performance, demonstrating its strength androbustness. Advancing Diagnostics: Geno1®’s innovative solution in liquid biopsy diagnostics sets it up as a future leader in precision medicine.
Pangaea Oncology
Barcelona, Spain
- Employees
-
We are specialists in finding these molecular alterations & in developing diagnostic technology, among others Liquid Biopsy. PANGAEA Oncology is a company focused on Precision Oncology, which offers both patients and pharmaceutical companies different services with the aim of improving the survival of cancer patients, their response to treatments, and their quality of life. The company has two main areas of activity interrelated, such as the clinical care division and the state-of-the-art laboratory that provides services in various areas, including molecular diagnostics as a reference laboratory for both patients and industry, pre-treatment services (clinic to industry), and diagnostic consultancy. PANGAEA ONCOLOGY’s mission is to change the natural history of the disease, improving the survival of cancer patients, through the application of the best professionals and technologies. To position PANGAEA ONCOLOGY as the reference in Europe in Precision Oncology, from patient treatment, the provision of services to the industry, the incorporation of disruptive technology and medical-scientific excellence, and directed towards systems intelligent IT and data exploitation (IA/BIG DATA). PANGAEA ONCOLOGY is a company focused on Precision Oncology, which offers both patients and pharmaceutical companies different services with the aim of improving the survival of cancer patients, their response to treatments, and their quality of life. Position PANGAEA ONCOLOGY as the reference in Southern Europe in Precision Oncology, from patient treatment, the provision of services to the industry, the incorporation of disruptive technology and medical-scientific excellence, and directed towards systems intelligent IT and data exploitation (IA/BIG DATA). Molecular companion diagnostics tests provide essential information that enables doctors to identify those patients that are most likely to benefit from a treatment.