Diaxxo AG, founded in 2020, is a spin-off of the Functional Materials Laboratory (FML) of ETH Zürich. Our mission is to enable DNA analyses to be widely diffused and adopted by making them accurate, fast and affordable. Our innovative cartridge makes PCR analysis faster and more convenient, with very short turnaround times. Load the DNA/RNA onto our cartridge (diaxxoPod), which comes preloaded with all the necessary reagents. Run the PCR test by means of our innovative device, that can perform extremely fast RT-qPCR. Thanks to our unique technology, we are able to carry out faster RT-PCR tests at lower cost, whilst ensuring the highest results accuracy and sensitivity.
Zurich, Switzerland
Founded in 2020
1-10 Employees
Startup
Working industry
Medical
Type of company
Manufacturer
Ownership structure
Public Company
Locations
1 Headquarter
Number of products
5 Products
Specialised areas
Medtech, Diagnostics, PCR, IVD, Molecular diagnostics, POC, Medical Device
Diaxxo AG offers a wide range of products and services
Product
Technology - Diaxxo
Go to product >
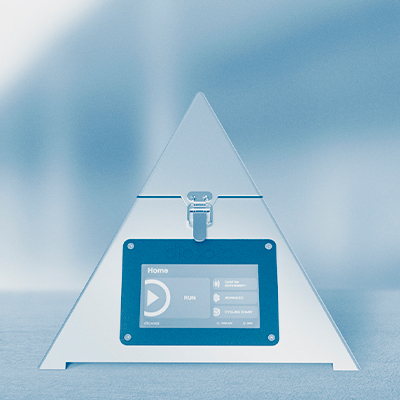
Product
All Products - Diaxxo
Go to product >
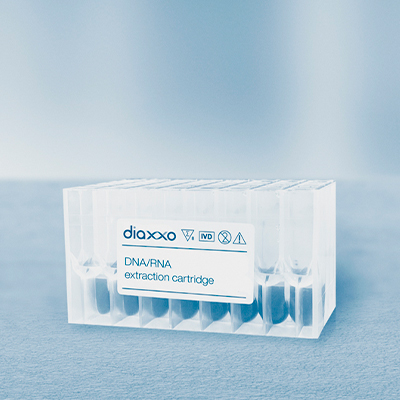
Product
Products - Extraction Cartridge - Diaxxo
Go to product >

Product
Products - Extractor - Diaxxo
Go to product >

Product
Products - diaxxoPCR - Diaxxo
Go to product >
An estimation about the ESG values based on digital data and signals. Important: The ESG scores are only based on information about the country, not the actual company itself
Country:
Switzerland
Overall risk estimation:
Very low
The ESG Data of countries are based on public sources
Environment
A
Grade (A-E)
View details
Social
A
Grade (A-E)
View details
Governance
A
Grade (A-E)
View details
Diaxxo AG operates in 1 country around the world
Get an overview of the locations of Diaxxo AG
Location
Country
State
City
Headquarter
Switzerland
Zurich
Zurich
Some frequent questions that have been asked about Diaxxo AG
Where is Diaxxo AG located?
The company headquarter of Diaxxo AG is located in Zurich, Zurich, Switzerland. It's worth noting, that the company may have more locations
How many employees does Diaxxo AG approximately have?
As of the latest available information Diaxxo AG has around 1-10 employees worldwide.
When was Diaxxo AG founded?
Diaxxo AG was founded in 2020
In which industries does Diaxxo AG mainly work?
The company Diaxxo AG has it's main focus in the industries of Medical
What is the current company status of Diaxxo AG?
Based on the founding year and the amount of employees the company Diaxxo AG seems to be a Startup at the current state. Note that over time that status can change
Check out some interesting alternative companies to Diaxxo AG
Dianax
Milan, Italy
11-50 Employees
2013
We are seeing a tectonic shift in healthcare infrastructure as we know it, powered by the smartphone revolution, and dianax is at the cutting-edge of mobile diagnostic transformation. The ongoing development of dianax lab-on-a-chip has been made possible thanks to investment from multiple sources. We are indebted to those in the European Commission who recognized early on the extraordinary potential of our technology in changing the face of diagnostic testing and awarded a significant grant through their Horizon 2020 Program. We are always happy to hear from interested parties. We have partnered with experts in rapid diagnostics – BioSpeedia (a spin-off of Institut Pasteur) – to bring to market a reliable test for rapid detection and differentiation of antibodies to novel coronavirus SARS-CoV-2. The COVID19SEROSpeed-IgM-IgG Rapid Test is now distributed by dianax in Italy, Belgium, Luxembourg, the Netherlands, Greece, Spain and Portugal. By bringing laboratory-grade disease screening, diagnosis and monitoring to the patient, dianax is taking diagnostics straight to the POINT OF NEED. While HbA1c-glycated hemoglobin blood testing for diabetes is our primary focus, the dianax lab-on-a-chip can test any protein extracted from bodily fluids (blood, urine, saliva), and so has the potential to transform diagnostic testing across a number of therapy areas.
Visby Medical
San Jose, United States
251-500 Employees
2012
We are a medical diagnostics company with portable PCR testing technology that may be implemented in both clinical and mobile laboratory settings. Watch our video to learn more about our mission and story. Click Diagnostics explores a number of markets and identifies sexual health as the direction to pursue. Small enough to fit in the palm of your hand, the PCR test delivers rapid and accurate results. NIH sponsors the FDA clinical study for the sexual health product. Our rapid PCR tests provide accurate and reliable results for healthcare providers all across the United States. In a world where speed and accuracy in disease testing has become increasingly important, the efficient protection of public health depends on the delivery of fast and reliable infectious disease testing to affected communities as quickly as possible. The Visby Medical portable, instrument-free PCR test delivers results in minutes, not days, making our PCR test kits a revolution in the molecular diagnostics industry.
Swift Molecular Diagnostics
Cambridge, United Kingdom
1-10 Employees
2016
Creating the next generation of lateral flow devices. SwiftDx’s portfolio of patented technologies enable ultra-boosted sensitivity lateral flow diagnostics for diseases which are undetectable with current lateral flow test technology. SwiftDx has successfully completed the initial technology development phase and is ready to commercialise its DNA detection and eAMP technologies. The company’s first product (the SwiftDx Mycoplasma Detection kit) has been launched and a further products are in development. Our goal is to better inform patients and healthcare practitioners with a new generation of lateral flow tests.
DiaDx-Liquid Biopsy for personalized cancer medicine
Montpellier, France
1-10 Employees
2015
We provide, with a simple blood draw, a multiparametric, qualitative and quantitative analysis of tumour-derived circulating DNA with unprecedented sensitivity level. DiaDx has built a strong expertise in all pre-analytical conditions revolving around an optimal circulating DNA analysis and follows its own stringent Standard Operating Procedures under Good Clinical Laboratory Practice (GCLP) guidelines. As such, IntPlex® provides a real-time analysis of the molecular evolution of the tumour providing information on the mutant clones dynamics during all phases of patient management care. IntPlex® is a sophisticated quantitative PCR-based method much more cost-effective (2 to 3-times) than any other competitor system.
FRIZ Biochem
Neuried, Germany
11-50 Employees
2004
With CYCLE® Dx we provide a unique solution for true Point-of-Care diagnostics. CYCLE® Dx provides the complete diagnostic workflow in a miniaturised environment and transfers Point-of-Care diagnostics to the next level:. Furthermore, we offer our customers state of the art oligonucleotides, phosphoramidites and nanoparticles.
DNA Electronics
London, United Kingdom
51-100 Employees
2003
Our mission is to bring dramatic, life-changing improvements to healthcare and beyond with fast, simple and scientifically sound products. DNAe Group Holdings LtdScale Space58 Wood Lane London W12 7RZ United Kingdom. At DNAe we are leading the way in rapid, near-patient sequencing testing that will revolutionize patient care for a range of unmet clinical needs. DNAe is developing the LiDia-SEQ™ Platform, a direct-from-specimen diagnostic system that performs DNA sequencing on a microchip, to provide rapid, actionable information to clinicians. We are creating radically different products that change the game for early diagnosis and monitoring of potentially fatal diseases. Regius Professor Chris Toumazou FRS, FREng, FMedSci founded the company in 2003, as a spin-out from Imperial College London, having invented a way of detecting protons released during DNA synthesis to enable DNA sequence detection using a standard silicon-chip based transistor. Regius Professor Chris Toumazou FRS, FREng, FMedSci founded the company in 2003, as a spin-out from Imperial College, London, having invented a way of detecting protons released during DNA synthesis to enable DNA sequence detection using a standard silicon-chip based transistor. The user-friendly system can operate in a variety of hospital environments at the point-of-need, with flexible levels of throughput to match a wide range of clinical demands.Our initial focus is on infectious disease diagnostics, where speed and microbial DNA sequence information can make the difference between life and death.