Our mission is to accelerate development of novel therapeutics by providing researchers with high-quality products and services for all stages of research. We are passionate about diving deep into the science to discover novel and innovative solutions for next-generation therapies. We are all about working with sponsors to help them overcome the challenges of their research. We provide a wide range of services to support early research and development through the clinical phase. We provide custom biodistribution, immunogenicity, pharmacokinetics, and biomarker assay services to support discovery through clinical phases. Experts in virology, immuno-oncology, cell and vector engineering, and assay development, our vision is to be a leader in research services and laboratory assays. This scientific approach to each project is what allows us to deliver excellence in contract research, custom products, and virus assays to support development of oncolytic virus therapies, gene and cell therapies, regenerative medicines, and vaccines. What makes Imanis stand out is our diverse scientific team of experts in virology, immuno-oncology, molecular biology, and assay development.
Rochester, United States
Founded in 2012
1-10 Employees
Working industry
Biotechnology
Type of company
Service provider, Manufacturer, Educational institution
Ownership structure
Privately Held
Locations
1 Headquarter
Number of products
16 Products
Specialised areas
Non-Invasive Imaging, Oncolytic Virotherapy, Contract Research Services, Reporter Gene Imaging, Genetics, Biotechnology, Medical
Imanis Life Sciences offers a wide range of products and services
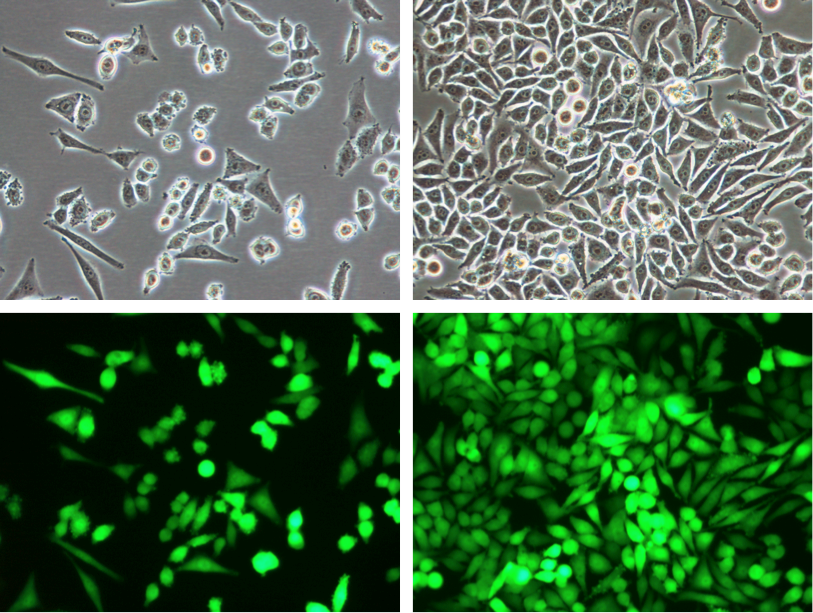
Product
Mel624-CMV-hNIS-Neo/eGFP-Puro Reporter Gene Cell Line - Imanis Life Sciences
Go to product >

Product
K562-Fluc-EmGFP - Imanis Life Sciences | United States
Go to product >

Product
LV-Fluc Reporter Gene Lentiviral Vector - Imanis Life Sciences
Go to product >

Product
HSV-GFP Reporter Gene Oncolytic Virus - Imanis Life Sciences
Go to product >

Product
Vaccinia vTF7-3 Reporter Gene Oncolytic Virus - Imanis Life Sciences
Go to product >

Product
Measles-RFP Reporter Gene Oncolytic Virus - Imanis Life Sciences
Go to product >

Product
PC3-eGFP-Neo - Imanis Life Sciences | United States
Go to product >
Product
4T1-mNIS (monoclonal) Reporter Gene Cell Line - Imanis Life Sciences
Go to product >
An estimation about the ESG values based on digital data and signals. Important: The ESG scores are only based on information about the country, not the actual company itself
Country:
United States
Overall risk estimation:
Low
The ESG Data of countries are based on public sources
Environment
D
Grade (A-E)
View details
Social
A
Grade (A-E)
View details
Governance
A
Grade (A-E)
View details
Imanis Life Sciences operates in 1 country around the world
Get an overview of the locations of Imanis Life Sciences
Location
Country
State
City
Headquarter
United States
Minnesota
Rochester
Some frequent questions that have been asked about Imanis Life Sciences
Where is Imanis Life Sciences located?
The company headquarter of Imanis Life Sciences is located in Rochester, Minnesota, United States. It's worth noting, that the company may have more locations
How many employees does Imanis Life Sciences approximately have?
As of the latest available information Imanis Life Sciences has around 1-10 employees worldwide.
When was Imanis Life Sciences founded?
Imanis Life Sciences was founded in 2012
In which industries does Imanis Life Sciences mainly work?
The company Imanis Life Sciences has it's main focus in the industries of Biotechnology
Check out some interesting alternative companies to Imanis Life Sciences
Iq Biosciences
Berkeley, United States
11-50 Employees
-
At iQ Biosciences, we provide focused research support to the pharmaceutical, biotechnology, and diagnostic industries in the form of intelligent research services and quality biospecimens. We are driven to go beyond being reliable – we excel at being a trusted partner in the biomedical community to help cure disease. We provide a number of assays to verify binding and specificity for your antibody or CAR-T cell therapeutic. We believe it’s urgent to help the biomedical research community deliver on therapeutic promises today. We believe all researchers should have access to the highest standards of testing services and investigative approaches for qualitatively assessing or quantitatively measuring their test compound’s bioactivity at practical prices. We specialize in designing and executing assays to determine this. It’s in our DNA to deliver comprehensive end-to-end service to our customers. So during project engagements, we keep you apprised of progress and provide a heads up on any warning signs.
BPS Bioscience Inc.
San Diego, United States
11-50 Employees
2005
We are experts in protein design, expression, purification, and characterization, cell line and lentivirus engineering, and biochemical and cellular assay development. Our products and services have enabled thousands of researchers to advance biological discoveries across a wide variety of disciplines, including epigenetics, cell signaling, SARS-CoV-2, immunotherapy, adoptive cell therapies, and many more. We are now ISO 9001:2015-certified, ensuring constant monitoring and continuous improvement in order to meet customer needs. We focus on emerging research needs and rapidly deliver solutions. We focus on developing quality products, providing excellent customer service, and maintaining a robust quality management system. These core values are at the heart of how we operate. Offered in a variety of key formats, our biochemical and cell-based assay kits are validated for screening inhibitors of your target of interest. We do the work to deliver you powerful results.
Alloy Therapeutics
Lexington, United States
101-250 Employees
2017
We are a team of 100+ people across 5 research sites, and our work is amplified by our ecosystem of hundreds of companies and thousands of engaged researchers. We offer our platforms and services to the entire drug discovery community and are committed to doing whatever it takes to find you the best therapeutics. Our commitment to reinvesting 100% of our revenue into innovation means we are always acquiring and developing new groundbreaking technologies to make you more successful. We offer foundational biologics discovery technologies through bespoke campaigns or through licensing into your labs. Our commitment to reinvesting 100% of our revenue into innovation means we are always developing new groundbreaking platforms and protocols to make our collaborators more successful. We offer an ever-growing stable of services and cutting-edge technology platforms to help you discover the best therapeutic antibodies in the ideal formats for your therapeutic targets. We offer foundational technologies and discovery capabilities that address the therapeutic index and delivery challenges that have historically limited the promise of antisense oligonucleotide (ASO) drug candidates. Keyway’s industry-leading solutions to TCRm/TCR-based therapeutic discovery including its proprietary specificity screening and high-quality pMHC complex antigen production capabilities 3.
Imquest Biosciences, Inc.
Frederick, United States
11-50 Employees
2004
We provide a customized, collaborative environment to solve complex research questions and are a trusted partner through all stages of preclinical development. ImQuest BioSciences provides preclinical contract research and development services to help academics, pharmaceutical and biotechnology companies develop new pharmaceuticals and biologics products. We have over 30 years of expertise in preclinical contract research and development services, a significant product development track record, and our team has extensive expertise in the development of small molecules, natural products, biologics and vaccines for the treatment and prevention of infectious disease, cancer, and inflammatory disease. ImQuest is a preclinical and infectious disease CRO that provides expert services to evaluate novel pharmaceutical products for the treatment and prevention of viruses, bacteria, cancer and inflammatory diseases.
BioInnovations
Mumbai, India
11-50 Employees
2010
We are committed to advancing the scientific understanding of complex therapeutic areas like Oncology, Immunology, Neurology, Infectious Disease, Regenerative Medicine, and the application areas like Microbiology, Genomics, Proteomics, Spatial Omics, Metabolomics, Polymers Analysis, and many more. We are proud of what we do as well as how we take care of the clients. We profusely thank BIOINNOVATIONS for assistance in the whole-genome sequence analysis. As a leader in the industry since 2010, We have an association with technology leaders for Clinical, Analytical, and Life Sciences requirements. We work to promote a healthier world by enabling our customers to deliver innovative technologies with a comprehensive portfolio from detection and imaging solutions to informatics and services. Honesty is the base of everything we do. Our core values define our business culture and who we are as BIOINNOVATIONS employees. To create a sustainable and high-value health system with Bioinnovations.
iCura Diagnostics LLC
Malvern, United States
1-10 Employees
2021
At iCura Diagnostics, we are revolutionizing the contract research organization (CRO) landscape by redefining the way laboratory testing and research services are delivered. Our mission is to provide agile, client-centric solutions that empower our partners to accelerate their precision medicine initiatives and bring life-saving therapies to patients faster. We are dedicated to provide this tool to a larger pool of researchers and healthcare providers. Our agile teams, comprised of experts and laboratory professionals, excel in the entire service life cycle. We specialize in emerging technologies and advanced laboratory testing solutions in areas crucial for new treatment development and validation, including immune-therapy, cell and gene therapy, spatial biology, tumor microenvironment, organoid culture systems, and more. As a vendor-agnostic CRO, our core mission is to help clients identify the most suitable laboratory testing platforms in a timely and cost-effective manner. Leveraging years of experience, we provide valuable insights into the strengths and limitations of each laboratory testing platform, particularly in the context of emerging tests. Navigating regulatory requirements is a crucial aspect of laboratory testing for clinical trials.